The Term Degenerate Is Used to Describe Atomic Orbitals Which
A maximum of two electrons each with its own spin quantum number s will occupy each of those orbitals. Electrons of an unbonded atom move around the atomic nucleus in orbitals.
Atomic And Molecular Orbitals Mse 5317
Degenerate orbitals Orbitals with identical energies.

. Alternatively atomic orbitals refer to functions that depend on the coordinates of one electron ie orbitals but are used as starting points for approximating wave functions that depend on the simultaneous coordinates. Degenerate orbitals are orbitals with the same energy. This leaves one unhybridised 2p orbital.
Molecular orbitals occur as pairs of degenerate same energy bonding and from TCHM 120 at SUNY Albany. Atomic orbital atomska orbitala. A cos θ occurs with degenerate sets of orbitals eg.
Firstly an electron is promoted from the 2s orbital to the 2p orbital. 100 4 ratings Option A and C is Correct Answer. Atomic orbitals atom Xs sorbital.
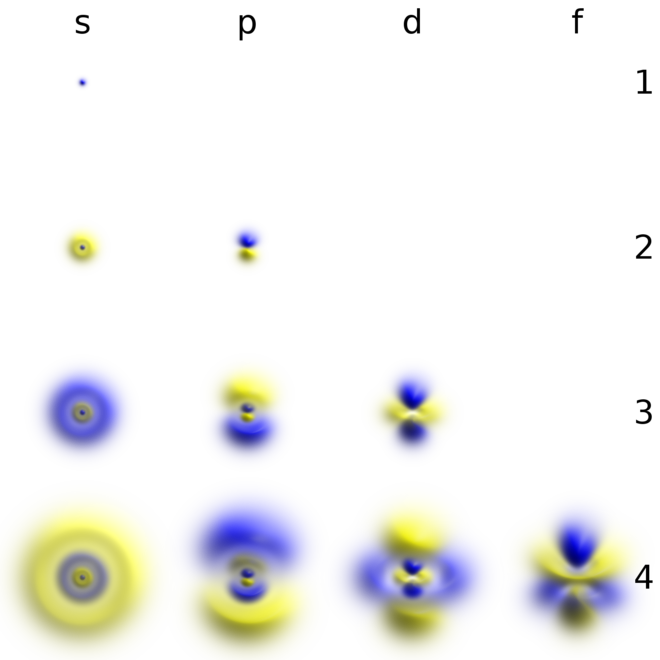
This page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as UK A level and its equivalents. They look differently in 3D-space but. D orbitals are described only in terms of their energy and f orbitals only get a passing mention.
This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atoms nucleus. A term used to describe atomic orbitals that have the same energy as each other d orbitals These have a value of l2 and occur in 5 different orientations corresponding to m-2-1012. They are different they may display differently in space around the nucleus but they are associated to the same energy.
Heres an example of the d-orbitals in chemistry. 02 Question 1 point Consider the following atomic orbitals and the definition of the term degenerate 1st attempt Which two of the following orbitals are degenerate. View the full answer.
Now you are given a colorred4d orbital and. Then on each carbon of the double bond the 2s orbital and two of the 2p orbitals combine to form three degenerate sp2 hybrid orbitals. Which pairs of sets of quantum numbers refer to spin paired electrons.
Write down ten sets of quantum numbers that describe the electrons in a degenerate set of 4d atomic orbitals. This degeneracy can sometimes be lifted by external electric or magnetic fields. A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular.
Degenerate orbitals are orbitals that have the same energy. Electron A negatively charged elementary particle of mass 9109390x10-31. The second shell can have a total of eight electrons for one s and three p.
Each atomic orbital can have maximum of two electrons. Degenerate orbitals are the orbitals of the same subshell of the same main shell. An atomic orbital is a mathematical term in atomic theory and quantum mechanics that describes the position and wavelike behaviour of an electron in an atom.
Ie 2Pz and 2Px. If the molecule has some symmetry the degenerate atomic orbitals with the same atomic energy are grouped in linear combinations called symmetry-adapted atomic orbitals SO which belong to the representation of the symmetry group so the wave functions that describe the group are known as symmetry-adapted linear combinations SALC. It explores s and p orbitals in some detail including their shapes and energies.
Each electron has its unique set of quantum numbers which means that two electrons can share one two or even three quantum numbers but never all four. There are only two electrons in the first shell as it has only s atomic orbitals. P x p x.
In atomic theory and quantum mechanics an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom. Hence these are 3 d x y 3 d z 2 3 d y z and 4 d x y 4 d y z 4 d z 2. Electron orbitals that have the same energy levels are called degenerate orbitals.
Write down ten sets of quantum numbers that describe the electrons in a degenerate set of 4d atomic orbitals. The orbitals are defined in terms of a fixed coordinate system The z-axis is selected so that it either coincides with the highest-fold rotation axis. Degenerate orbitals are orbitals that have the same energy.
A total of 10 sets of quantum numbers can be used here. Degeneration in chemistry means a set of energy-levels like atomic orbitals that exhibit the same energy but have a different shape andor orientation in space. Option 3 - Filling each p orbital with 1 electron with mixed spins before returning to complete each orbital n 2 l 1 ml -1 ms ½ n 2 l 1 ml 0 ms - ½ Orbitals of the same energy - Hunds Rule For degenerate orbitals remember degenerate means equal energy the orbitals will fill to maximise the electron spin-filling each degenerate orbital with one electron with the.
You can break this degeneracy by applying a suitable external field on the system electric or magnetic field for example. Degenerate orbitals are the orbitals. As you know we use four quantum numbers to describe the position and spin of an electron in an atom.
1 2 3 A fundamental principle of these theories is that as atoms bond to form molecules a certain number of. Atomic orbitals can be the hydrogen-like orbitals which are exact solutions to the Schrödinger equation for a hydrogen-like atom ie an atom with one electron.
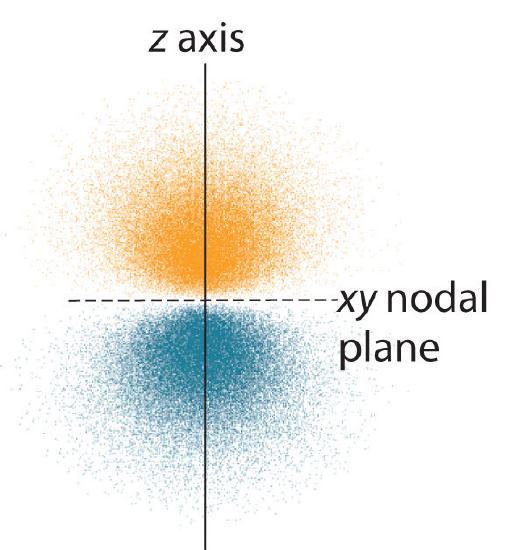
7 6 The Shape Of Atomic Orbitals Chemistry Libretexts
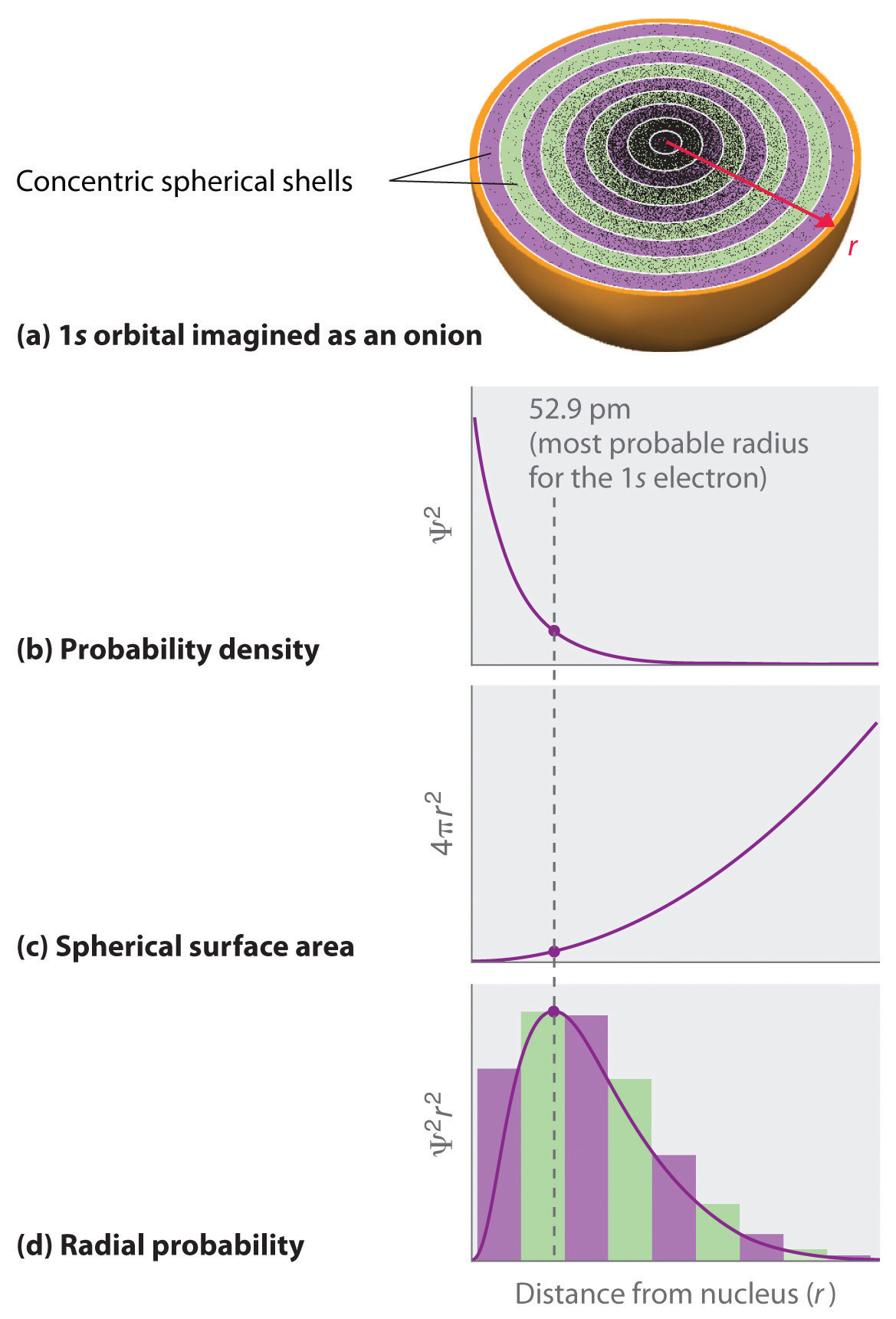
1 4 Atomic Orbitals And Their Energies Chemistry Libretexts
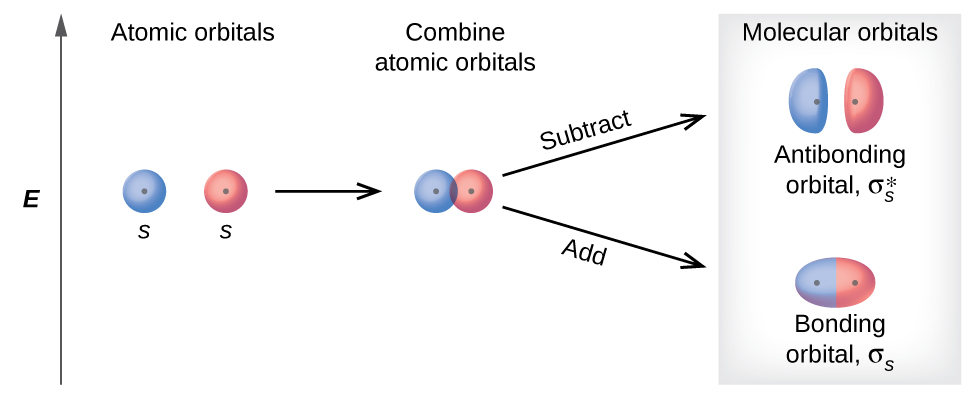
3 1 Molecular Orbital Theory Chemistry Libretexts
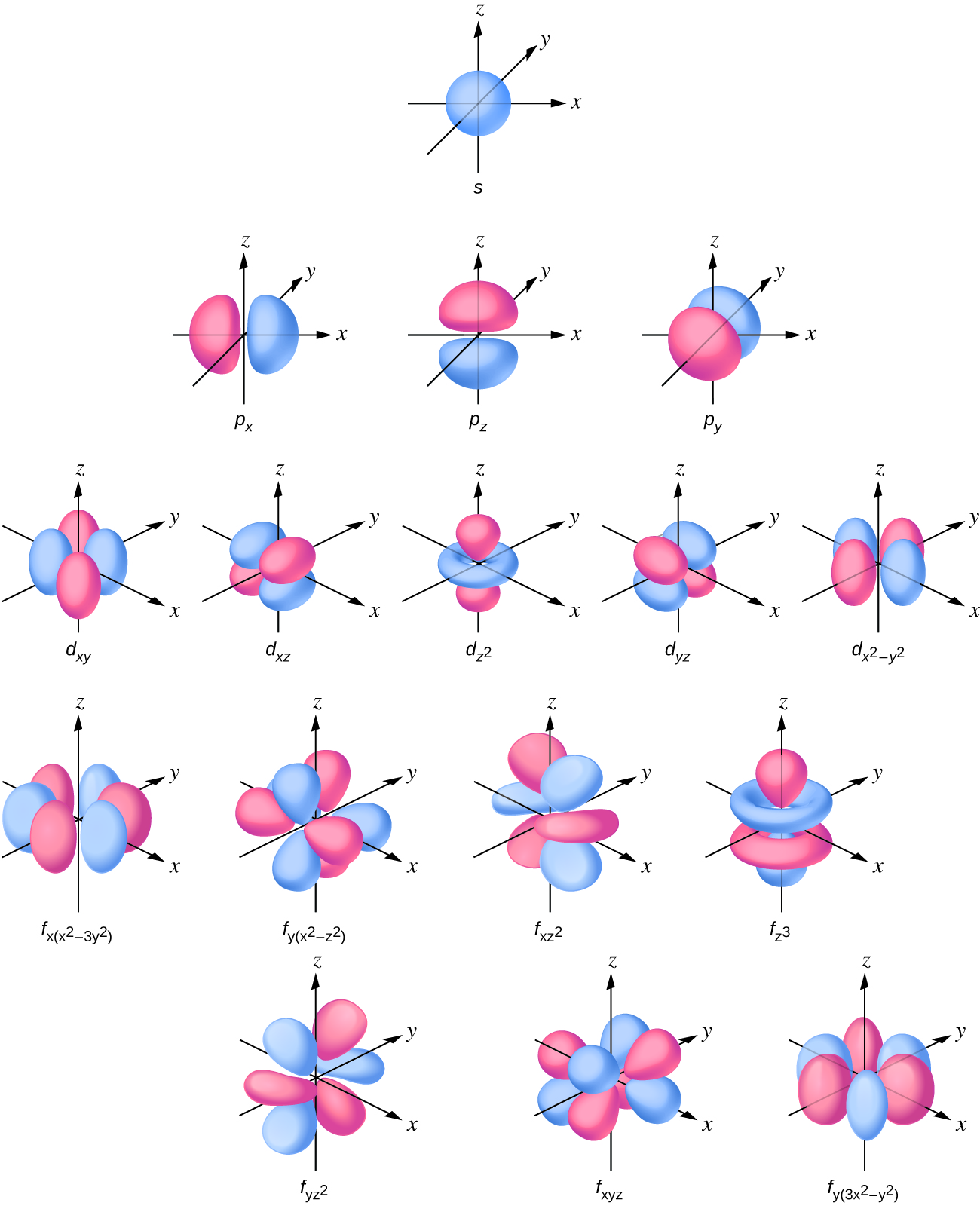
2 2 Atomic Orbitals And Quantum Numbers Chemistry Libretexts

Orbital Chemistry And Physics Britannica

Simple Explanation Of Orbitals Chemistry Stack Exchange

Quantum Mechanics Why Do Non Hydrogen Atomic Orbitals Have The Same Degeneracy Structure As Hydrogen Orbitals Physics Stack Exchange
Valence Bond Theory And Hybrid Orbitals Introductory Chemistry 1st Canadian Edition Clone
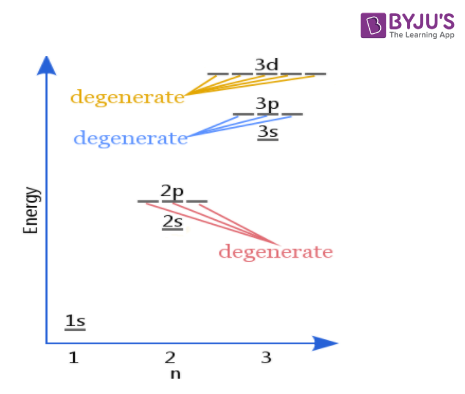
Degenerate Orbitals Explanation With Diagram Examples On Byju S

Atomic Orbital An Overview Sciencedirect Topics

Chemical Bonding Shapes Of Atomic Orbitals Britannica
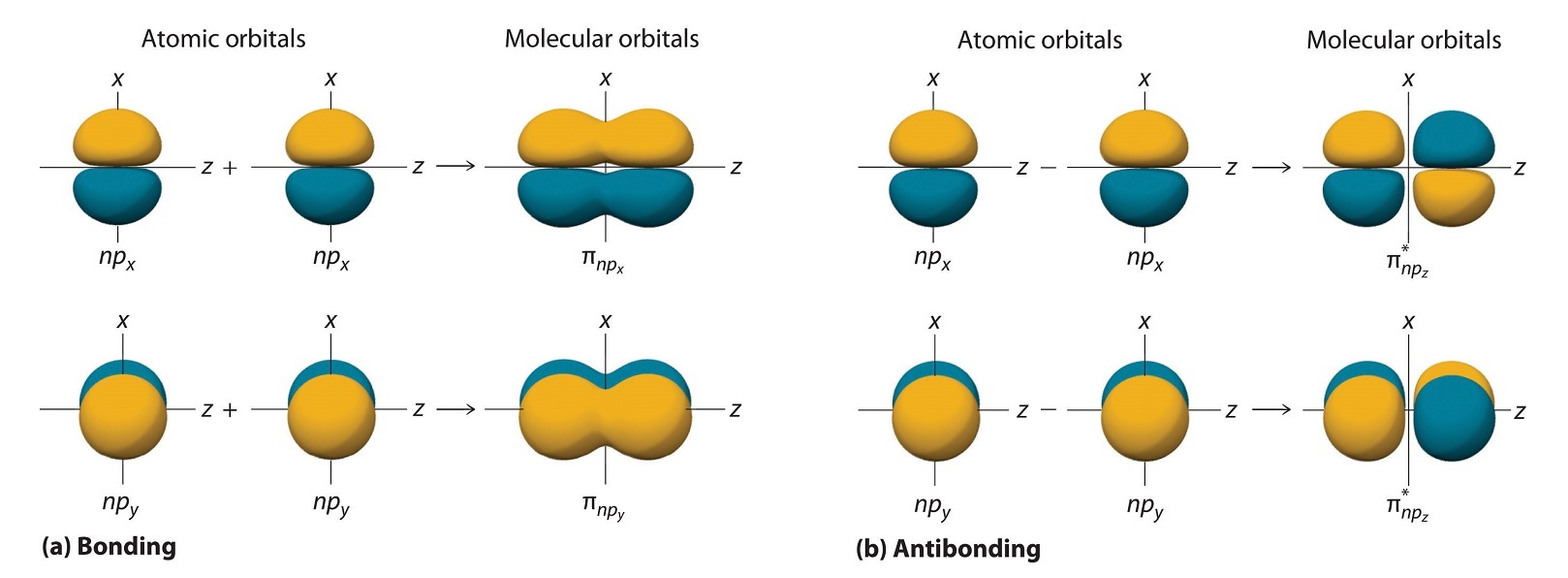
27 Molecular Orbitals With Higher Energy Atomic Orbitals Extra Lecture Chemistry Libretexts
Why Is It That The Number Of Atomic Orbitals Used To Generate Molecular Orbitals Is Equal To The Number Of Molecular Orbitals Produced From Those Atomic Orbitals Quora
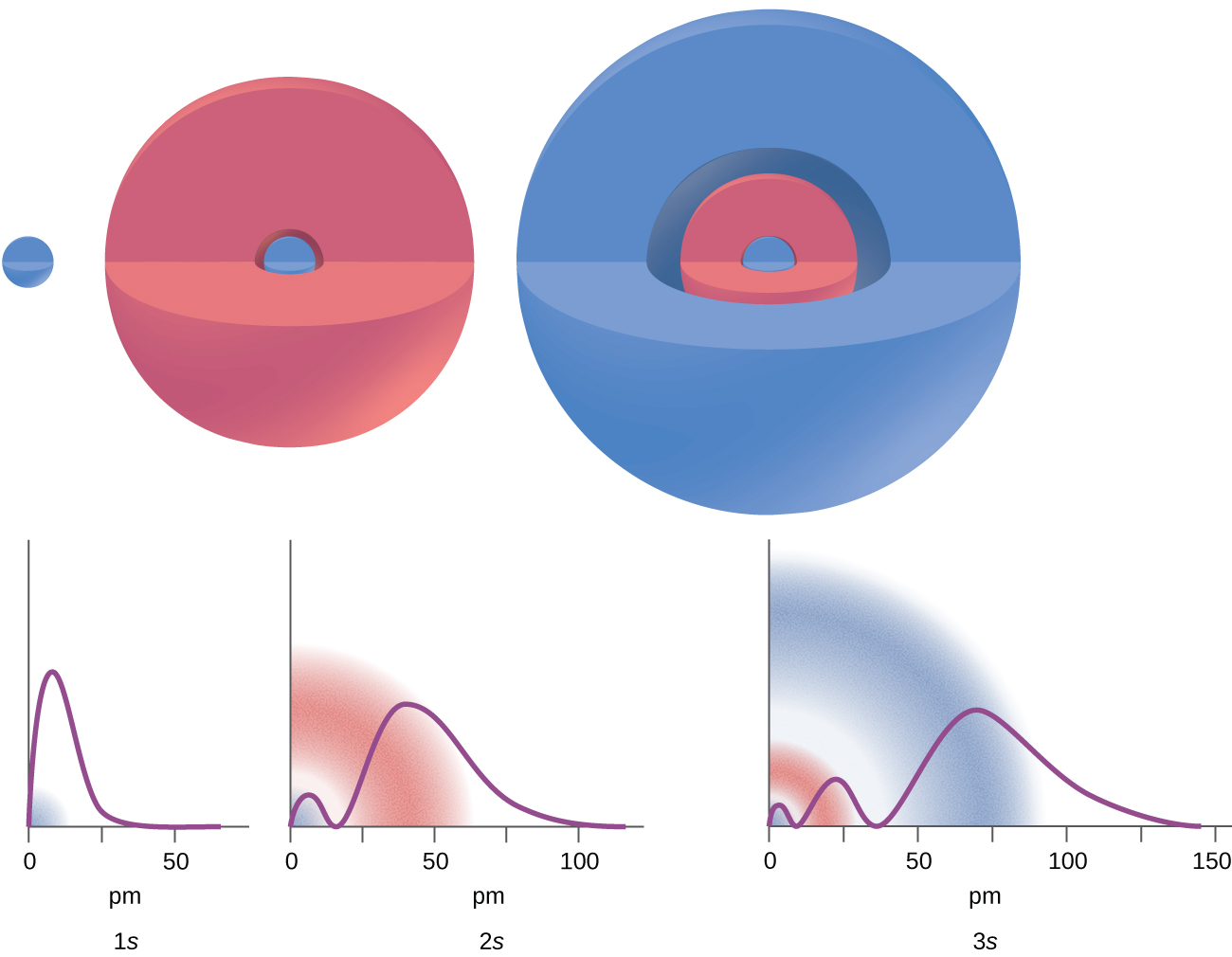
2 2 Atomic Orbitals And Quantum Numbers Chemistry Libretexts
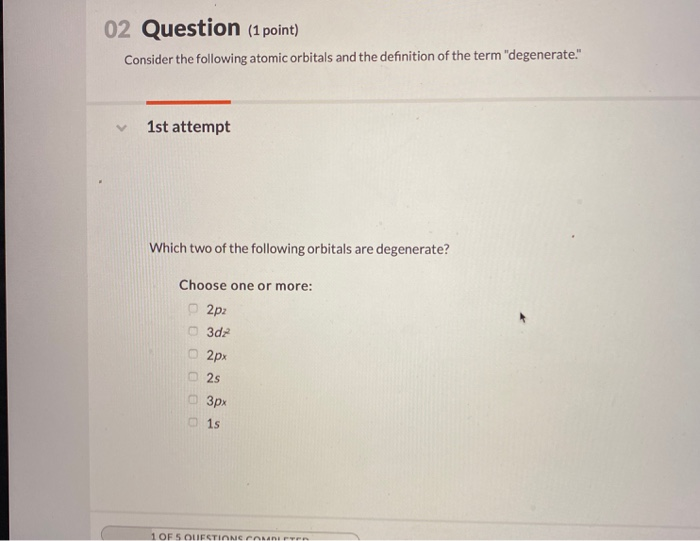
Solved 02 Question 1 Point Consider The Following Atomic Chegg Com


Comments
Post a Comment